roElut™ LLE+ (Liquid-Liquid Extraction)
Classical liquid-liquid extraction using a separation funnel is often associated with certain disadvantages such as formation of emulsion, poor phase separation, high solvent consumption, low degree of automation, and high personnel cost. However, liquid-liquid extraction is more efficient using ProElut™ LLE+ cartridges. The simple and excellent performance of ProElut™ LLE+ cartridges eliminates emulsions and therefore, results in higher recoveries and cleaner extractions.
How does ProElut™ LLE+ work?
The aqueous sample is applied to the ProElut™ LLE+ sorbent. The sample then distributes itself in the form of a thin film over the chemically inert matrix and thus acts as a stationary phase. Subsequently, elution takes place using organic solvents that are nonmiscible with water, e.g. diethyl ether, ethyl acetate or halogenated hydrocarbons. All the lipophilic substances are extracted from the aqueous into the organic phase. During this process the aqueous phase remains on the stationary phase. The eluate is free from emulsions and can be evaporated for further analysis.
Application of ProElut™ LLE+
ProElut™ LLE+ has been widely applied in the sample preparation of urine, whole blood, plasma, serum, gastric juice, liquor, amniotic fluid, and animal and plant tissue. Other applications are in the areas of environmental and residue analysis, e.g. the analysis of industrial and domestic wastewater.
.png)
ProElut™ Diatomaceous Earth Filter Aid
• Improves extraction efficiency
• Adsorbs moisture from samples
Diatomaceous earth is used as a filter aid to improve extraction efficiency of densely packed soils, such as clays. By mixing the sample with diatomaceous earth, recoveries can be improved and excess moisture can be absorbed.
Determination of Aromatic Amines Originating from Azo Dyes
1. Application
Determination of aromatic amines originating from azo dyes in textile products
2. Preparation
Cut samples into approximately 5 x 5 mm small pieces, and weigh out 1.0 g sample. Add 16 mL 70 ± 2 ºC citrate buffer (0.06 M, PH 6)in a reactor, seal the reactor and shake it until the sample is immersed into the liquid. Keep the reactor in a thermostatic water bath for 30 min at 70 ± 2 ºC. Add 3.0 mL sodium dithionite solution into the reactor, seal and shake immediately, then keep the reactor in thermostatic water bath for 30 min at 70 ± 2 ºC, take it out, and cool it down to room temperature with warm water.
3. Final Extraction
Open the reactor and press the sample by a glass stick, then transfer the liquid to ProElut™ AZO (Cat#62551), let stand for 15 min. Wash the reactor 4 times with 20 mL diethy ether, collect all solutions and transfer to ProElut™ AZO for extraction. Use a control flow rate, and collect the elution solution into a 250 mL flat bottom flask.
4. Concentration
The elution solution is concentrated in a vacuum to 1 mL at 35 ºC. Dry the concentrated liquid by N2, and dissolve the residue by 1 mL MeOH. Use HPLC or GC / MS to analyze.
5. GC / MS Method
Column: DM-5MS 30 m x 0.25 mm x 0.25 μm (Cat#8221)
Injection: Splitless, 250 ºC
Sample: Aromatic amines, 1 μL
Carrier gas: He (> 99.999%)
Flow rate: 0.6 mL/min,
Temperature program: 50 ºC (0.5 min) to 150 ºC at 20 ºC/min (hold 8 min) to 230 ºC at
20 ºC/min (hold 20 min) to 260 ºC at 20 ºC/min (hold 5 min)
MS Condition
Interface temperature: 270 ºC, Scan range: 35 - 350 amu, Ionizaion: EI @ 70 eV
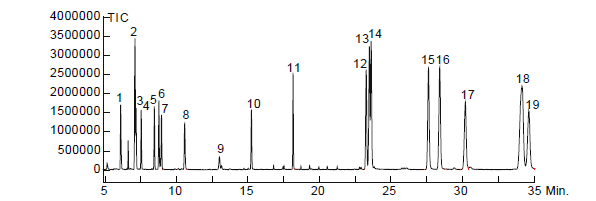
1. o-Toluidine
2. 2,4-Dimethylaniline
3. o-Anisidine
4. 4-Chloroaniline
5. 2-Methoxy-5-methylaniline
6. 2,4,5-Trimethylaniline
7. 4-Chloro-2-methylaniline
8. 2,4-Diaminotoluene
9. 2, 4-Diaminoanisole
10. 2-Aminonaphthalene
11. 4-Aminobiphenyl
12. 4,4’-Oxydianiline
13. Benzidine
14. 4,4’-Methylenedianiline
15. 3,3’-Dimethyl-4,4’-diaminodiphenylmethane
16. 3,3’-Dimethoxybenzidine
17. 4,4’-Thiodianiline
18. 3,3’-Dichlorobenzidine
19. 3,3’-Dimethoxybenzidine




















.png)

